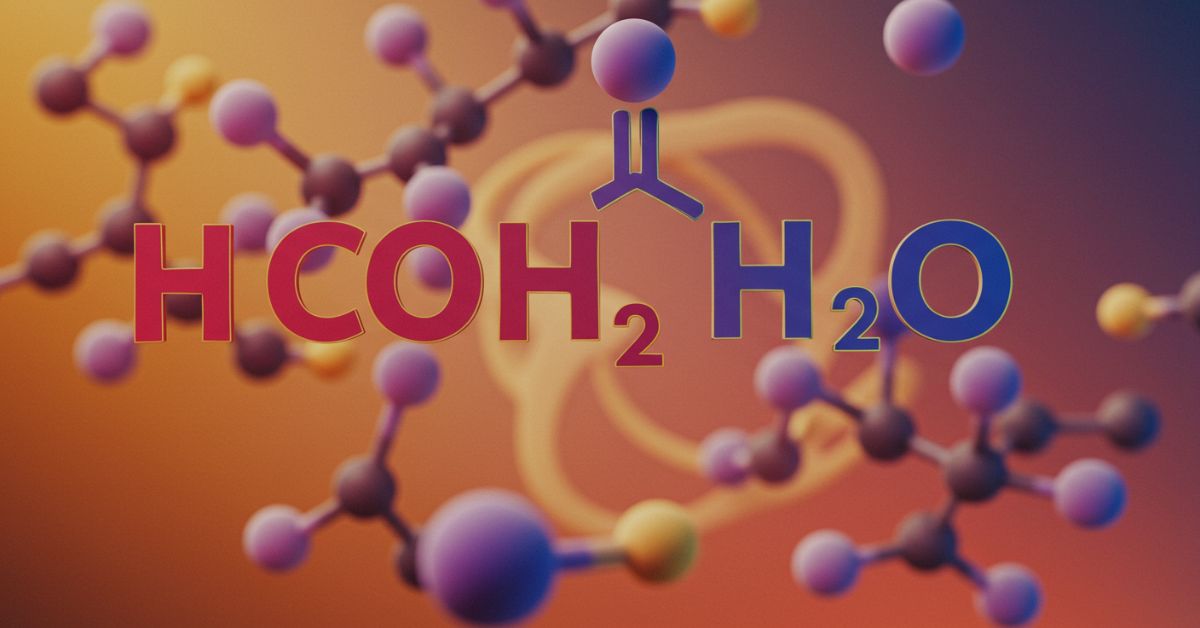
Contents
- 1 Introduction
- 2 Understanding the Chemical Structure of hcooch ch2 h2o
- 3 Physical and Chemical Properties of hcooch ch2 h2o
- 4 Synthesis and Production of hcooch ch2 h2o
- 5 Chemical Reactions Involving hcooch ch2 h2o
- 6 Applications of hcooch ch2 h2o in Industry
- 7 Safety Considerations and Handling Precautions
- 8 Regulatory and Environmental Impact
- 9 Frequently Asked Questions (FAQs)
- 9.1 Q1: What is the primary function of hcooch ch2 h2o?
- 9.2 Q2: Is hcooch ch2 h2o soluble in water?
- 9.3 Q3: Can hcooch ch2 h2o be used in food products?
- 9.4 Q4: What safety measures should be taken when handling hcooch ch2 h2o?
- 9.5 Q5: How does hcooch ch2 h2o react under high temperatures?
- 9.6 Q6: What are the industrial alternatives to hcooch ch2 h2o?
- 10 Conclusion
Introduction
Understanding chemical compounds and their behavior is crucial in chemistry and various industrial applications. One such compound is hcooch ch2 h2o, which has notable properties and reactions that make it valuable in organic chemistry. In this comprehensive guide, we will explore the chemical structure, physical and chemical properties, synthesis, practical applications, potential hazards, and safety measures of hcooch ch2 h2o. Additionally, we will discuss its reactivity, industrial applications, regulatory considerations, and frequently asked questions.
Understanding the Chemical Structure of hcooch ch2 h2o
Molecular Formula and Structure
The molecular formula hcooch ch2 h2o suggests a combination of different functional groups that influence its reactivity. Breaking it down:
- HCOOCH represents a formate or ester functional group.
- CH2 is a methylene (-CH2-) group, which can act as a bridge between different chemical entities.
- H2O (water) often plays a role in hydrolysis reactions, solubility, and stability.
Structural Representation
A more detailed structural representation of hcooch ch2 h2o helps in understanding how atoms are bonded and interact with other substances in chemical reactions.
Bonding and Electron Configuration
- The ester bond in the molecule contributes to its reactivity.
- Polarity and hydrogen bonding influence its solubility and chemical interactions.
Physical and Chemical Properties of hcooch ch2 h2o
Physical Properties
- Appearance: Depending on its composition, it may exist as a clear liquid or a crystalline solid.
- Molecular Weight: The precise molecular weight depends on its structural arrangement.
- Boiling and Melting Points: These properties vary based on intermolecular forces and purity.
- Solubility: Highly soluble in polar solvents like water and alcohols due to possible hydrogen bonding.
Chemical Properties
- Acid-Base Behavior: Depending on its structure, hcooch ch2 h2o may exhibit weak acidic properties.
- Reactivity with Water: Potential hydrolysis reactions may occur, leading to breakdown products.
- Combustibility: If it contains organic components, it may be flammable.
- Oxidation-Reduction Reactions: Can participate in redox reactions under the right conditions.
- Thermal Stability: The compound decomposes under extreme heat.
Synthesis and Production of hcooch ch2 h2o
Laboratory Synthesis
The synthesis of hcooch ch2 h2o can be achieved through controlled chemical reactions involving formate esters, methylene intermediates, and aqueous conditions. A general process includes:
- Reacting formic acid with an alcohol to form the ester component.
- Introducing methylene groups through organic synthesis techniques.
- Hydration or hydrolysis steps to ensure proper stability of the final compound.
Industrial Production
For large-scale applications, the compound can be synthesized through:
- Catalytic reactions that improve yield and purity.
- Biochemical processes involving enzyme-mediated transformations.
- Thermal or pressure-assisted reactions that optimize conversion efficiency.
- Electrochemical Synthesis methods for improved sustainability.
Factors Affecting Production Efficiency
- Temperature and pressure conditions
- Reagent concentration and purity
- Catalyst selection for optimization
Chemical Reactions Involving hcooch ch2 h2o
Hydrolysis Reaction
Hcooch ch2 h2o can undergo hydrolysis in the presence of water, breaking down into smaller organic components.
Esterification
If the compound contains ester groups, it can react with acids or alcohols to form new esters, which are useful in fragrance and pharmaceutical industries.
Oxidation Reactions
Under oxidative conditions, hcooch ch2 h2o may produce carboxylic acids or other oxidation products, depending on the reagents used.
Reduction Reactions
Hydrogenation or reduction of hcooch ch2 h2o can lead to the formation of alcohols or simpler hydrocarbons.
Polymerization Potential
The presence of methylene groups suggests the potential for polymerization reactions, which may be useful in material science.
Applications of hcooch ch2 h2o in Industry
Pharmaceutical Industry
- Used in the synthesis of medicinal compounds.
- Can act as an intermediate in drug formulation.
Chemical Manufacturing
- Utilized in organic synthesis for producing esters and other derivatives.
- Plays a role in polymer production.
Food and Fragrance Industry
- May contribute to flavor or aroma compounds.
- Potential use in food additives.
Agricultural Applications
- Used in pesticide formulation.
- Enhances soil treatment chemicals.
Environmental Applications
- Involved in biodegradable chemical synthesis.
- Used in water purification techniques.
Safety Considerations and Handling Precautions
Potential Hazards
- Flammability: If volatile, handle with caution near open flames.
- Toxicity: Exposure to hcooch ch2 h2o in high concentrations may be harmful.
- Reactivity: Avoid mixing with strong acids or bases without proper control.
- Carcinogenic or Mutagenic Potential: Must be evaluated in industrial applications.
Safe Handling Guidelines
- Use personal protective equipment (PPE) such as gloves and safety glasses.
- Store in a cool, dry place away from incompatible substances.
- Dispose of waste following environmental regulations.
Regulatory and Environmental Impact
Government Regulations
- FDA & EPA Compliance: Must meet safety regulations.
- Occupational Safety Standards: Proper exposure limits must be defined.
Environmental Impact
- Biodegradability: Can it be broken down naturally?
- Toxicity in Water Bodies: Effects on aquatic ecosystems.
Frequently Asked Questions (FAQs)
Q1: What is the primary function of hcooch ch2 h2o?
Answer: hcooch ch2 h2o is mainly used in organic synthesis, pharmaceutical development, and industrial chemical production.
Q2: Is hcooch ch2 h2o soluble in water?
Answer: Yes, due to its molecular structure, it shows significant solubility in water and other polar solvents.
Q3: Can hcooch ch2 h2o be used in food products?
Answer: If approved by regulatory agencies, it may be used in specific food or fragrance formulations.
Q4: What safety measures should be taken when handling hcooch ch2 h2o?
Answer: Ensure proper ventilation, wear protective gear, and follow chemical safety guidelines.
Q5: How does hcooch ch2 h2o react under high temperatures?
Answer: It may undergo decomposition, releasing gases or other breakdown products.
Q6: What are the industrial alternatives to hcooch ch2 h2o?
Answer: Alternatives include similar esters or biodegradable organic compounds with comparable properties.
Conclusion
The compound hcooch ch2 h2o has significant importance in chemistry due to its diverse applications, chemical properties, and reactivity. Understanding its synthesis, reactions, and safety precautions allows researchers and industry professionals to use it effectively. Whether applied in pharmaceuticals, industrial production, or research laboratories, mastering the properties and handling of hcooch ch2 h2o is essential for safe and efficient utilization.
By following best practices and chemical guidelines, hcooch ch2 h2o can be optimized for various applications, ensuring both productivity and safety in its use.
Ethan Cole is a versatile writer at hsnime.co.uk, offering fresh perspectives and engaging content across various topics. With a passion for creativity and knowledge, Ethan aims to provide insightful articles that resonate with a diverse audience.